
Intra-Cellular Therapies Inc
NASDAQ:ITCI

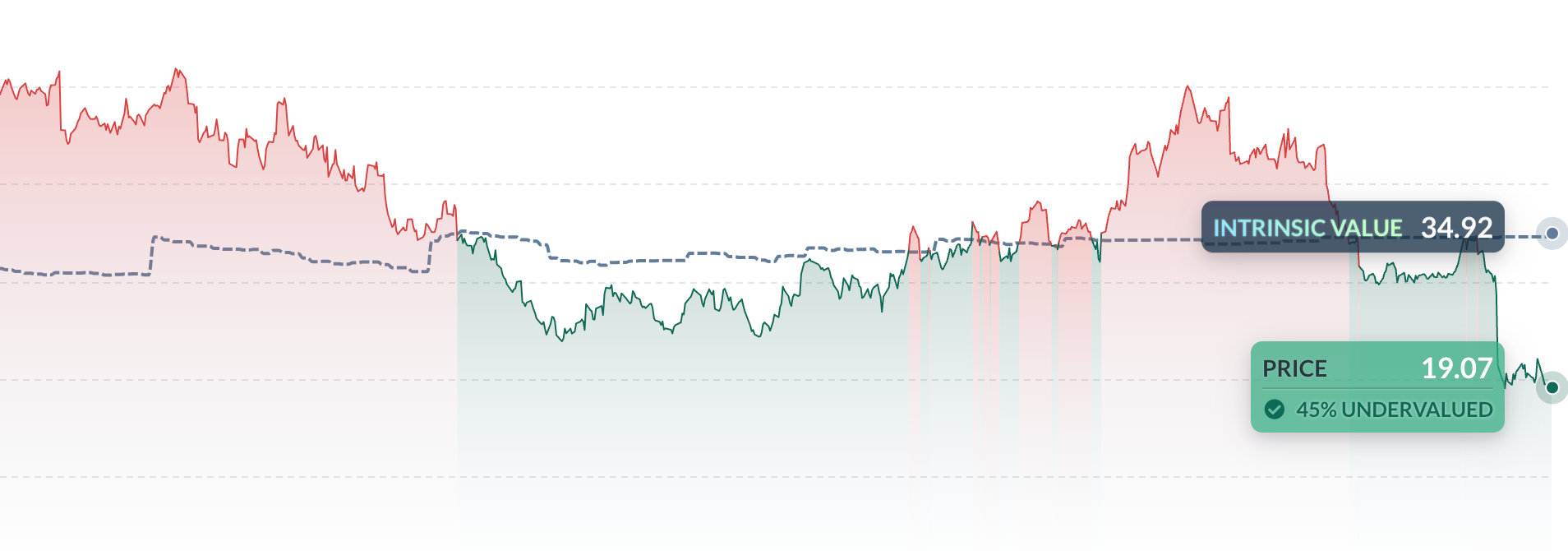
Intrinsic Value
The intrinsic value of one
 ITCI
stock under the Base Case scenario is
90.4
USD.
Compared to the current market price of 131.87 USD,
Intra-Cellular Therapies Inc
is
Overvalued by 31%.
ITCI
stock under the Base Case scenario is
90.4
USD.
Compared to the current market price of 131.87 USD,
Intra-Cellular Therapies Inc
is
Overvalued by 31%.
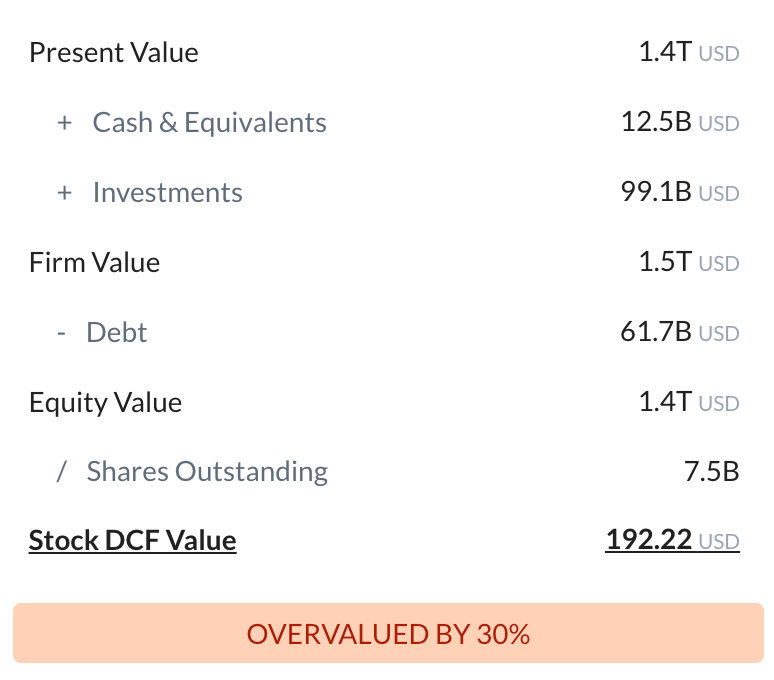
The Intrinsic Value is calculated as the average of DCF and Relative values:

Valuation History
Intra-Cellular Therapies Inc

Fundamental Analysis

Heightened reliance on CAPLYTA leaves the firm vulnerable if commercial uptake underperforms — with almost all revenues riding on a single product, any shift in physician prescribing habits or unexpected regulatory snags could sharply impact top-line results.
CAPLYTA’s strong efficacy-benign side effect profile in schizophrenia and bipolar depression positions it as a potential best-in-class therapy — positive feedback from both patients and clinicians could accelerate market adoption and boost revenue growth.

Revenue & Expenses Breakdown
Intra-Cellular Therapies Inc

Balance Sheet Decomposition
Intra-Cellular Therapies Inc

| Current Assets | 1.3B |
| Cash & Short-Term Investments | 1B |
| Receivables | 166.5m |
| Other Current Assets | 139.8m |
| Non-Current Assets | 59.5m |
| PP&E | 14.9m |
| Other Non-Current Assets | 44.7m |
Free Cash Flow Analysis
Intra-Cellular Therapies Inc

| USD | |
| Free Cash Flow | USD |
Earnings Waterfall
Intra-Cellular Therapies Inc

|
Revenue
|
680.9m
USD
|
|
Cost of Revenue
|
-57m
USD
|
|
Gross Profit
|
623.9m
USD
|
|
Operating Expenses
|
-740.6m
USD
|
|
Operating Income
|
-116.7m
USD
|
|
Other Expenses
|
42m
USD
|
|
Net Income
|
-74.7m
USD
|
ITCI Profitability Score
Profitability Due Diligence

Intra-Cellular Therapies Inc's profitability score is 38/100. The higher the profitability score, the more profitable the company is.

Score
Intra-Cellular Therapies Inc's profitability score is 38/100. The higher the profitability score, the more profitable the company is.
ITCI Solvency Score
Solvency Due Diligence

Intra-Cellular Therapies Inc's solvency score is 81/100. The higher the solvency score, the more solvent the company is.

Score
Intra-Cellular Therapies Inc's solvency score is 81/100. The higher the solvency score, the more solvent the company is.
Wall St
Price Targets
ITCI Price Targets Summary
Intra-Cellular Therapies Inc

According to Wall Street analysts, the average 1-year price target for
 ITCI
is 133.21 USD
with a low forecast of 119.18 USD and a high forecast of 138.6 USD.
ITCI
is 133.21 USD
with a low forecast of 119.18 USD and a high forecast of 138.6 USD.
Dividends

Current shareholder yield for  ITCI is
.
ITCI is
.
Shareholder yield represents the total return a company provides to its shareholders, calculated as the sum of dividend yield, buyback yield, and debt paydown yield. What is shareholder yield?

The intrinsic value of one
 ITCI
stock under the Base Case scenario is
90.4
USD.
ITCI
stock under the Base Case scenario is
90.4
USD.
Compared to the current market price of 131.87 USD,
 Intra-Cellular Therapies Inc
is
Overvalued by 31%.
Intra-Cellular Therapies Inc
is
Overvalued by 31%.





















































 You don't have any saved screeners yet
You don't have any saved screeners yet